| 1 | Molar enthalpy of vaporization (The Clausius-Clapeyron equation) | -438s.png) |
| 2 | Partial pressure of solute in Henry\'s law (given molar concentration of solute,Henry\'s law constant) | -337s.png) |
| 3 | Partial pressure of solute in Henry\'s law (given mole fraction,Henry\'s law constant) | -338s.png) |
| 4 | Partial pressure of solute in Henry\'s law (given molar concentration of solute,Henry\'s law constant) | -339s.png) |
| 5 | Henry\'s law constant at temperature T (given Henry constant at standard temperature,enthalpy of solution) | -340s.png) |
| 6 | Henry\'s law constant at temperature T (given Henry constant at standard temperature,enthalpy of solution) | -341s.png) |
| 7 | Henry\'s law constant in geophysics (given excess chemical potentials of solute gas in two phases) | -342s.png) |
| 8 | Number concentration of solute gas in melt phase (Henry\'s law in geophysics,chemical potential) | -343s.png) |
| 9 | Number concentration of solute gas in gas phase (Henry\'s law in geophysics,chemical potential) | -344s.png) |
| 10 | Chemical potential of a component in ideal solution by Raoult\'s law (given mole fraction) | -345s.png) |
| 11 | Gibbs free energy change of mixing by Raoult\'s law (given mole fractions) | -346s.png) |
| 12 | Mole fraction of a component in solution by Raoult\'s law and Dalton\'s law | 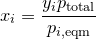 |
| 13 | Mole fraction in non-ideal solution by Raoult\'s law,ideal-gas law (fugacity,activity coefficient) | -348s.png) |
| 14 | Partial pressure in non-ideal solution by Raoult\'s law,ideal-gas law(fugacity,activity coefficient) | -349s.png) |
| 15 | Enthalpy change for a single reaction (Hess' law) | -421s.png) |
| 16 | Entropy change for a reaction (Hess' law) | -422s.png) |
| 17 | Gibbs free energy change for a reaction (Hess' law) | -423s.png) |
| 18 | Change in Gibbs free energy (given change in enthalpy, temperature and change in entropy) | -424s.png) |
| 19 | Standard change of reaction in Gibbs free energy (given temperature and equilibrium constant) | -425s.png) |
| 20 | Change of reaction in Gibbs free energy (Given standard change of reaction in Gibbs free energy, temperature and reaction quotient) | -426s.png) |
| 21 | Change in Gibbs free energy (given number of electrons per mole product,Faraday constant and electrode potential) | -427s.png) |
| 22 | Electrode potential of the reaction (standard electrode potential of the reaction, number of electrons per mole product and reaction quotient) | -428s.png) |
| 23 | Equilibrium constant at absolute temperature (Van 't Hoff equation) | -429s.png) |
| 24 | Equilibrium constant | 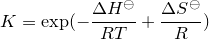 |
| 25 | Half-cell reduction potential (Nernst equation) | -431s.png) |