| 26 | Partial pressure of solute in Henry\'s law (given molar concentration of solute,Henry\'s law constant) | -337s.png) |
| 27 | Partial pressure of solute in Henry\'s law (given mole fraction,Henry\'s law constant) | -338s.png) |
| 28 | Partial pressure of solute in Henry\'s law (given molar concentration of solute,Henry\'s law constant) | -339s.png) |
| 29 | Henry\'s law constant at temperature T (given Henry constant at standard temperature,enthalpy of solution) | -340s.png) |
| 30 | Henry\'s law constant at temperature T (given Henry constant at standard temperature,enthalpy of solution) | -341s.png) |
| 31 | Henry\'s law constant in geophysics (given excess chemical potentials of solute gas in two phases) | -342s.png) |
| 32 | Number concentration of solute gas in melt phase (Henry\'s law in geophysics,chemical potential) | -343s.png) |
| 33 | Number concentration of solute gas in gas phase (Henry\'s law in geophysics,chemical potential) | -344s.png) |
| 34 | Chemical potential of a component in ideal solution by Raoult\'s law (given mole fraction) | -345s.png) |
| 35 | Gibbs free energy change of mixing by Raoult\'s law (given mole fractions) | -346s.png) |
| 36 | Mole fraction of a component in solution by Raoult\'s law and Dalton\'s law | 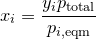 |
| 37 | Mole fraction in non-ideal solution by Raoult\'s law,ideal-gas law (fugacity,activity coefficient) | -348s.png) |
| 38 | Partial pressure in non-ideal solution by Raoult\'s law,ideal-gas law(fugacity,activity coefficient) | -349s.png) |
| 39 | Percentage of Calcium by permanganometry | 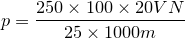 |
| 40 | Percent crude protein by Kjeldahl method | 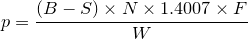 |
| 41 | Percentage of silica and sand by Ash | 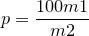 |
| 42 | Percentage of crude fibre by Ash | 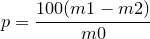 |
| 43 | Percentage of chloride as sodiumchloride by Argentometry | 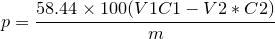 |
| 44 | Equivalent weight of acid | 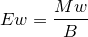 |
| 45 | Normality of acid | 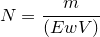 |
| 46 | Equivalent weight of oxidizing agents | 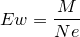 |
| 47 | Henderson-Hasselbalch equation | 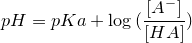 |
| 48 | Another form of Henderson-Hasselbalch equation | 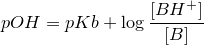 |
| 49 | Enthalpy | 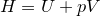 |
| 50 | Mass fraction | 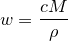 |